Click here to download a video notes sheet before watching the following video.
Cation exchange capacity (CEC) is defined as the sum total of the exchangeable cations that a soil can adsorb. Exchangeable cations are positively charged ions (e.g., Na+, Ca2+, K+) held on or near the negatively charged surfaces of soil particles and which may be replaced by other positively charged ions in the soil solution. Since they are readily exchanged with other cations, they are also readily available to plants. Cation exchange is, together with photosynthesis, a fundamental life-supporting process and without it terrestrial ecosystems would not be able to retain sufficient nutrients to support plant growth.
Cation exchange capacity is measured by one of several standard methods where all adsorbed cations in a soil are replaced by a common ion (such as NH4+) and then the amount of adsorbed common ion is determined.

Choice of a method to determine CEC is highly pH dependent:
- Ammonium chloride (NH4Cl); effective CEC, at pH of sample
- Sodium acetate (NaOAC) pH 8.2
- Ammonium acetate (NH4OAc) pH 7
- Barium chloride (BaCl2); appropriate for acid soils
Click here to download a video notes sheet before watching the following video.
Calculation
CEC is commonly reported as meq/100g. Note that these units are equivalent to cmol(+)/kg (centimoles of positive charge per kg) and either unit is acceptable.
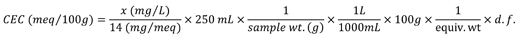
x = Sample reading (mg/L)
d.f. = dilution factor used
References and Resources
- Hendershot, W.H., H. Lalande, and M. Duquette. 2008. Ion exchange and exchangeable cations. In Carter, M.R., and E.G. Gregorich (eds). Soil Sampling and Methods of Analysis. 2nd ed. Canadian Society of Soil Science, CRC Press and Taylor & Francis Group. Oxford, UK.