The type of exchangeable cations associated with soil particles differ from one climatic region to another – Ca2+, Al3+, and H+ being most prominent in humid regions, while Ca2+, Mg2+, and Na+ dominate in semi-arid and arid regions. Extractable cations of primary interest are: calcium (Ca2+), magnesium (Mg2+), potassium (K+), and sodium (Na+). Potassium is an essential nutrient that is required in relatively large amounts by plants. Calcium and Mg are required in concentrations of about one-tenth of that of K. Sodium is not considered to be an essential element for most plant species, but Na concentration in soil can be an important indicator of salinity/alkalinity and it can have a negative impact on soil structure.
Potassium, Ca, and Mg present in the soil on the cation exchange complex usually correlate well with plant uptake of these elements by plants. The determinations of exchangeable Ca, Mg, K, and Na are done following replacement of these ions by a cation such as ammonium (NH4+).
Depending on the purpose and desired strength of extraction, there are different choices for extracting cations. The following are a list of options (from weakest to strongest):
- Morgan extract (sodium acetate and acetic acid)
- EDTA (ethylenediaminetetraacetic acid)
- DTPA (Diethylenetriaminepentaacetic acid) – appropriate for near neutral or calcareous soils
- Ammonium acetate (NH4OAc)
- 1 M hydrochloric acid (HCl)
Note that, depending on the procedure chosen, the first extraction solution from a cation exchange capacity (CEC) analysis can sometimes be used for cation determinations. Other elements of potential interest that could be measured from these extracts are: aluminum (Al), iron (Fe), manganese (Mn), and zinc (Zn)
Analysis of extracts can be done by atomic absorption (AA) or by inductively coupled plasma optical emission spectroscopy (ICP-OES) or inductively coupled plasma mass spectrometry (ICP-MS).
Click here to download a video notes sheet before watching the following video.
Calculation
Results are generally reported as meq/100 g soil. Note that these units are equivalent to cmol(+)/kg (centimoles of positive charge per kg) and either unit is acceptable.
-
If instrument readings are reported in meq/L
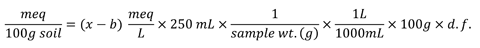
x = Sample reading (meq/L)
b = Blank reading (meq/L)
d.f. = dilution factor -
If instrument readings are reported in ppm (e.g. mg/L)
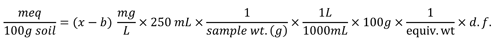
x = Sample reading (ppm or mg/ L)
b = Blank reading (ppm or mg/L)
d.f. = dilution factor
equiv wt. = equivalent weight (mg/meq)
References and Resources
- Haby, V.A., M.P. Russelle, and E.O. Skogley. 1990. Testing soils for potassium, calcium, and magnesium. In Westerman, R.L. (ed). Soil Testing and Plant Analysis. 3rd edition. ASA-SSSA, Madison, WI.
- Hendershot, W.H., H. Lalande, and M. Duquette. 2008. Ion exchange and exchangeable cations. In Carter, M.R., and E.G. Gregorich (eds). Soil Sampling and Methods of Analysis. 2nd ed. Canadian Society of Soil Science, CRC Press and Taylor & Francis Group. Oxford, UK.
- Martens, D.C., and W.L. Lindsay. 1990. Testing soils for copper, iron, manganese, and zinc. In Westerman, R.L. (ed). Soil Testing and Plant Analysis. 3rd edition. ASA-SSSA, Madison, WI.
- Soltanpour, P.V., G.W. Johnson, S.M. Workman, J.B. Jones, and R.O. Miller. 1996. Inductively coupled plasma emission spectrometry and inductively coupled plasma-mass spectroscopy. In Sparks, D.L. (ed). Methods of Soil Analysis: chemical methods. Part 3. Soil Sci. Soc. Am. Book Series No. 5. ASA-SSSA, Madison, WI.
- Wright, R.L., and T.I. Stuczynski. 1996. Atomic absorption and flame emission spectrometry. In Sparks, D.L. (ed). Methods of Soil Analysis: chemical methods. Part 3. Soil Sci. Soc. Am. Book Series No. 5. ASA-SSSA, Madison, WI.